Robotic Assisted Nerve Grafting to Relieve Compensatory Sweating
In the polite form we say that people perspire. In a little less polite form but well understood – people sweat. As is usually the case in medicine there is a precise term, hidrosis. Excessive sweating is hyperhidrosis. At one time or another everybody sweats too much. The body perspires as a means of cooling itself, so high temperatures and humidity may cause some people to sweat excessively.
Thermography for efficacy of nerve grafting
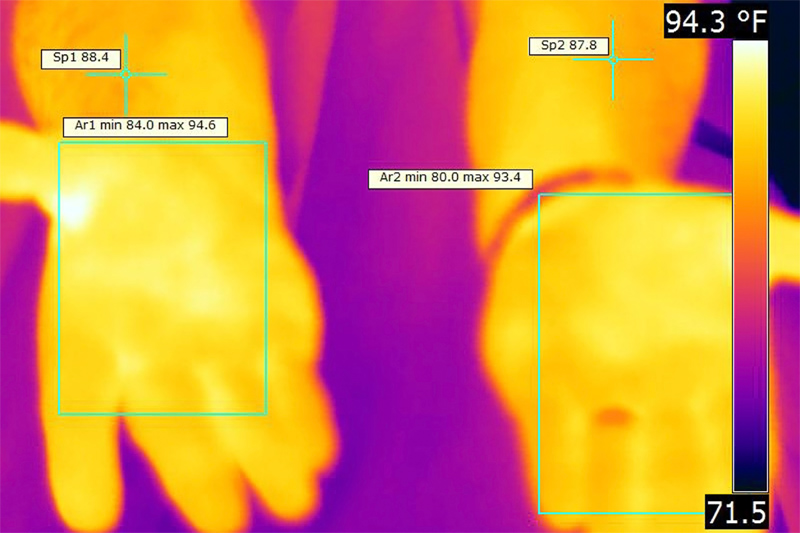
Stress and physical exertion can also make people sweat too much. These are natural reactions with obvious causes that are typically momentary and not a medical problem. Excess sweating, hyperhidrosis, can become a medical problem when it occurs in unusual locations, in unusual amounts, or under unusual circumstances. For example, literally millions of people sweat a great deal through the palms of their hands (palmar hyperhidrosis). It can be so bad that they are afraid to shake hands, hold paper documents, play musical instruments or touch water sensitive electronics. Because this and other forms of hyperhidrosis can seriously affect people’s lives, it can be a medical condition subject to treatment.
Treatments run a gamut from prescription antiperspirants, sweat gland blockers, Botox injections, to surgery. In more severe cases, especially with extraordinarily sweaty palms, a specific surgery called an endoscopic thoracic sympathectomy is frequently recommended. This procedure cuts, burns or clamps a section of a primary chain of nerves in the spine called the sympathetic nerve. This nerve bundle is part of the system that controls automatic bodily responses, for example, sweating in response to fear or threat. The sympathetic nerve connects to many sweat glands, including those in the hands. A thoracic sympathectomy prevents the function of a specific nerve segment just below the neck (usually near the thoracic vertebra position T4). This procedure has a track record of bringing relief to patients with excessively sweaty hands of around 85-95%. The flip side of this excellent success rate is that side effects are relatively frequent, anywhere from 20-80% of cases. The most common of these side effects is called compensatory sweating.
Compensatory sweating generally means that if treatment stops sweating in one place such as the palms, then somewhere else on the body one or more other locations such as the groin start excessive sweating. This is not exactly like perspiration whack-a-mole, but there is the sense the body is attempting to compensate for a perceived loss of temperature control by sweating more somewhere else. While compensatory sweating is not necessarily a significant problem, in a small percentage of cases it is severe enough to impair quality of life – sometimes worse than the original case of hyperhidrosis. In a number of these patients, there is a strong desire to reverse the effects of the sympathectomy.
A Nerve Graft Procedure to Reverse a Sympathectomy
An original endoscopic thoracic sympathectomy (ETS) is a relatively delicate procedure, as are most nerve surgeries. Reversing an ETS procedure is even more complex and delicate. The object of a sympathectomy reversal is to repair the sympathetic nerve by inserting a graft from another nerve. If the graft ‘takes,’ the sympathetic nerve may regenerate to some extent and the compensatory sweating and associated conditions may decrease or even disappear.
When considering robotic assisted surgery, the first phase of the surgical procedure includes methods of identifying the sites and extent of the previous sympathectomy. The initial procedure is performed with a thorascope and involves the surgeons searching for the burn marks or the clipped area on the sympathetic chain and the relationship of that area with the associated nerves connecting to other areas of the body (sympathetic ganglia). The condition of this area may determine if a restoration is possible.
Team Approach to Research, Development and Application
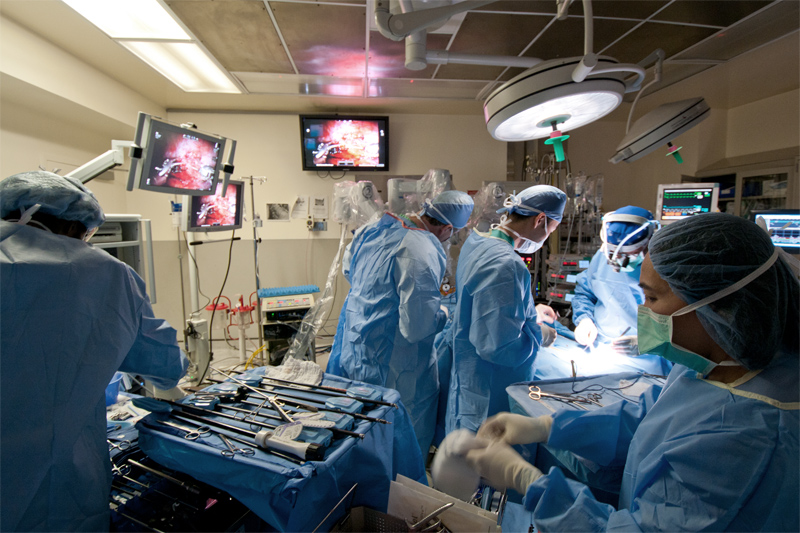
Once the previous sympathectomy is identified and a decision is made whether to proceed with reversal attempt, the second phase usually begins with opening three to five surgical ‘ports’ (incisions) about the size of a dime that are used for access to the thoracic cavity by various robotic instruments and the high resolution thoracoscopic video camera. Next, if a sympathectomy reversal is feasible then an appropriate graft needs to be procured. The nerve type used for sympathectomy reversal is typically an intercostal nerve (a portion of the spinal nerve) or a sural nerve (from the lower portion of the leg). The size and makeup of the sural nerve makes it a popular choice for both motor and sensory nerve reconstructions. As the sympathetic chain likely has a combination of fibers that are similarly sized, the sural nerve seems like an appropriate choice for graft. On the other hand, an intercostal nerve serves well for the transplant because it can be harvested using the same ports in the chest cavity and on the outside, is almost the same size as the sympathetic nerve chain.
There are different side effects and benefits for each type of graft, and the choice is usually discussed in a preoperative interview with the patient. Regardless of the type of nerve graft selected, the segment must be carefully separated and then dissected free from the surrounding tissue that holds the nerve chain in place.
The third phase attaches the ends of the graft nerve to the ends of the sympathetic nerve. Alignment is important as are the incredibly small sutures (stitches) used to attach the nerve segments. When the graft is in place, the supporting tissue (pleura) is reconnected and the access ports are closed to finish the operation.
Robotic Assisted Nerve Grafting
Although nerve grafting is the gold standard for repairing damaged or in this case intentionally disabled nerves, it requires careful work at two surgical locations – the donor site, where a compatible nerve provides tissue for the graft, and the recipient site, which must be prepared for the graft and then connected to the transplant tissue. Both surgical locations involve very small elements, as nerve (neuron) components are often measured in micrometers (millionths of a meter) and tissue segments in scant millimeters (thousandths of a meter). These small dimensions are usually beyond the limit of human eye and hand capability, even where magnifying video is used. In contrast, robotically controlled instruments are miniaturized and designed to work at the micro (micrometer) level. This sort of procedure is usually called microsurgery.
Minimally Invasive Micro-surgery
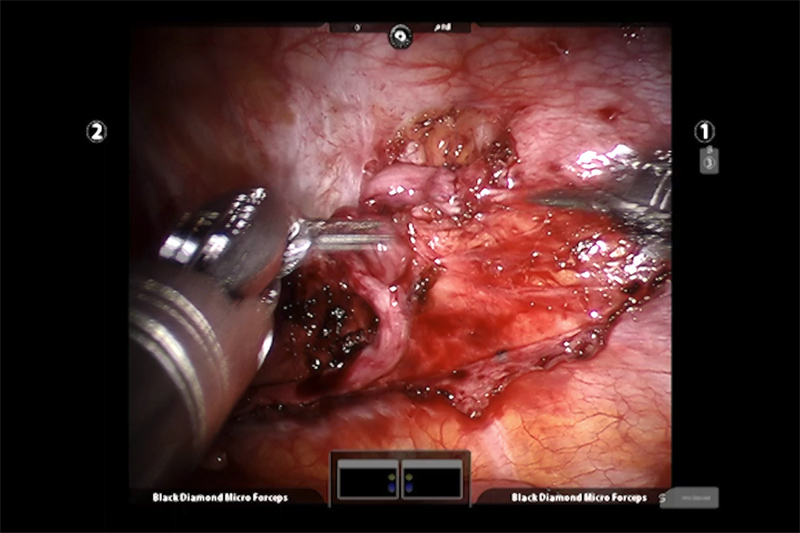
Robotically assisted surgery does not mean surgery by a robot; far from it. The surgeon sits at a computerized console that looks like a shrouded video arcade game device and operates the arms and instruments that carry out the procedure. The surgeon is constantly assisted by the computers and mechanics of the robotic system but remains in full control every step of the way.
Next to the patient is a specialized cart that supports the four robotic arms of the system. Three of these arms can wield a variety of surgical instruments; the fourth arm is for a highly specialized three-dimensional (3-D) camera controlled by the surgeon. A combination of computer and mechanics translates the surgeon’s hand movements at the console into smooth, tremor free and appropriately scaled movement of the instruments that work inside the patient. That means the surgeon’s movements are adjusted by the da Vinci system to be appropriately small and of just the right force for the procedure.
Between the magnified and exceptionally clear view of the surgical field (work area) and the super-fine control of the miniature surgical instrument, using this system allows the surgeon to perform very complex maneuvers in very small spaces, for example suturing the intercostal graft into the sympathetic nerve in this procedure.
The goal for the robotic assisted approach is true minimally invasive surgery, meaning that with very small access points to the body, the use of miniaturized surgical instruments, and the ability to perform ultra-precise surgical movements in a very small space – the patient suffers a minimum of trauma. This translates into less pain, decreased chance of infection, faster recovery and a shorter hospital stay.
Limitations to This Procedure
The limitations to robotic assisted nerve grafting – and reversing a sympathectomy – have little to do with the use of robotics or the da Vinci Surgery System. It has mostly to do with the nerves and the nervous system. While a thoracic sympathectomy has proven to be effective treatment for some kinds of excessive sweating, how exactly, this works isn’t fully understood. Likewise, when a sympathectomy results in the body starting to sweat copiously at another location, compensatory sweating; the cause of this change isn’t fully understood. Consequently it should not be surprising that the results of a graft to reverse a sympathectomy are not always fully predictable. The procedure is still relatively new and there are questions about the permanency of the graft, the regeneration of appropriate nerve signals, and whether the body will develop other side effects.